BNC210 (Negative Allosteric Modulator)
BNC210 is Bionomics’ proprietary molecule in development for the treatment of Social Anxiety Disorder (SAD), Post-Traumatic Stress Disorder (PTSD) and other anxiety disorders.
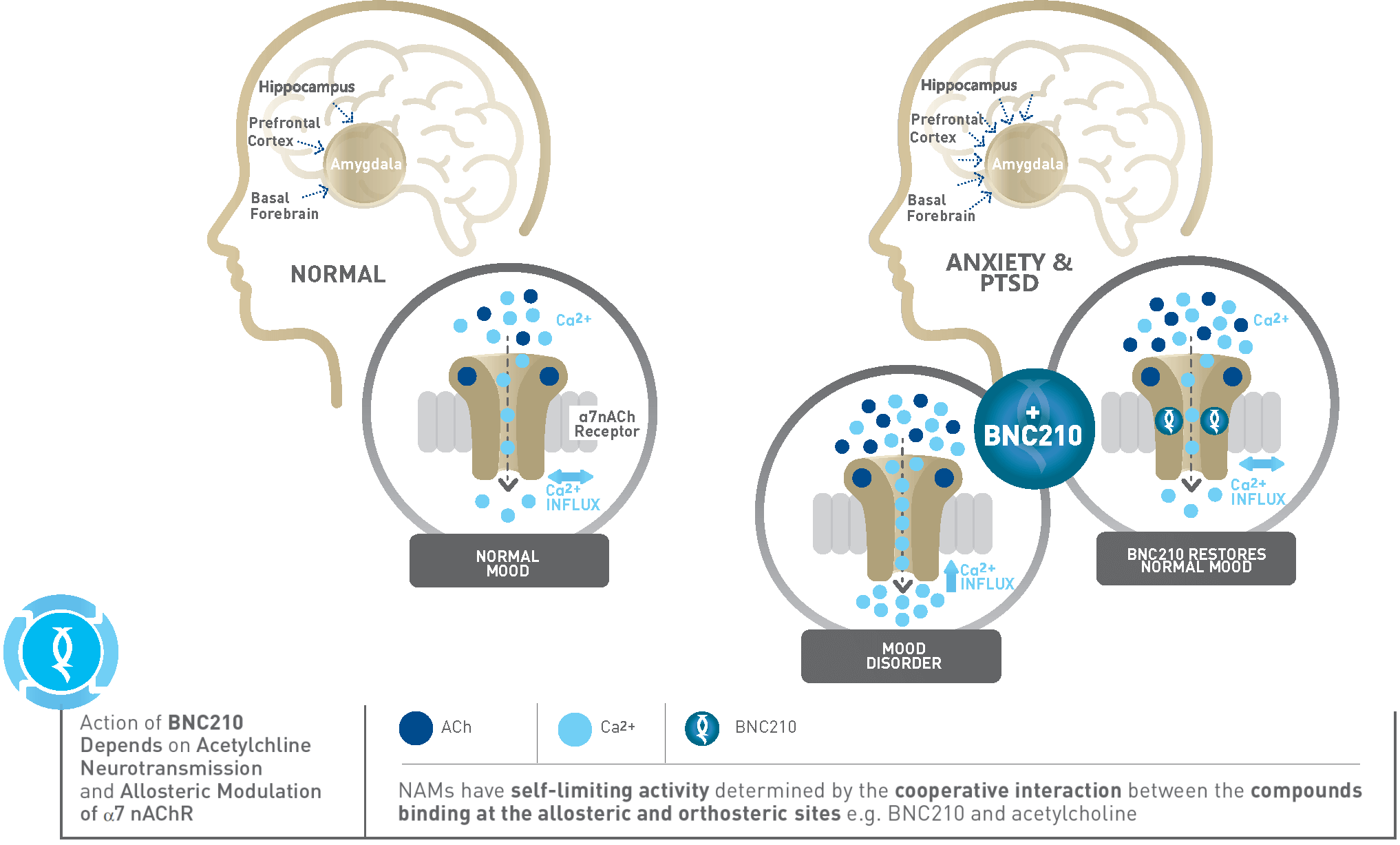
As a negative allosteric modulator of the alpha 7 nicotinic acetylcholine receptor (α7 nAChR), BNC210 has a unique and differentiated mechanism of action and extensive nonclinical and clinical studies have shown it has several properties which may be useful to treat SAD, PTSD and neuropsychiatric disorders.
BNC210 Holds the Potential to Addresses the Shortcomings of Existing Therapies
There is a significant need for improved therapeutics for SAD and PTSD with superior efficacy, response rates, fewer side effects and a faster onset of action, which we believe may be achieved by targeting a unique mechanism of action with BNC210.
BNC210 has also demonstrated a well-tolerated safety profile and no food effect, indicating additional potential advantage over of the current standard of care for SAD, PTSD and other anxiety disorders (e.g. antidepressants, benzodiazepines).
Social Anxiety Disorder (SAD)
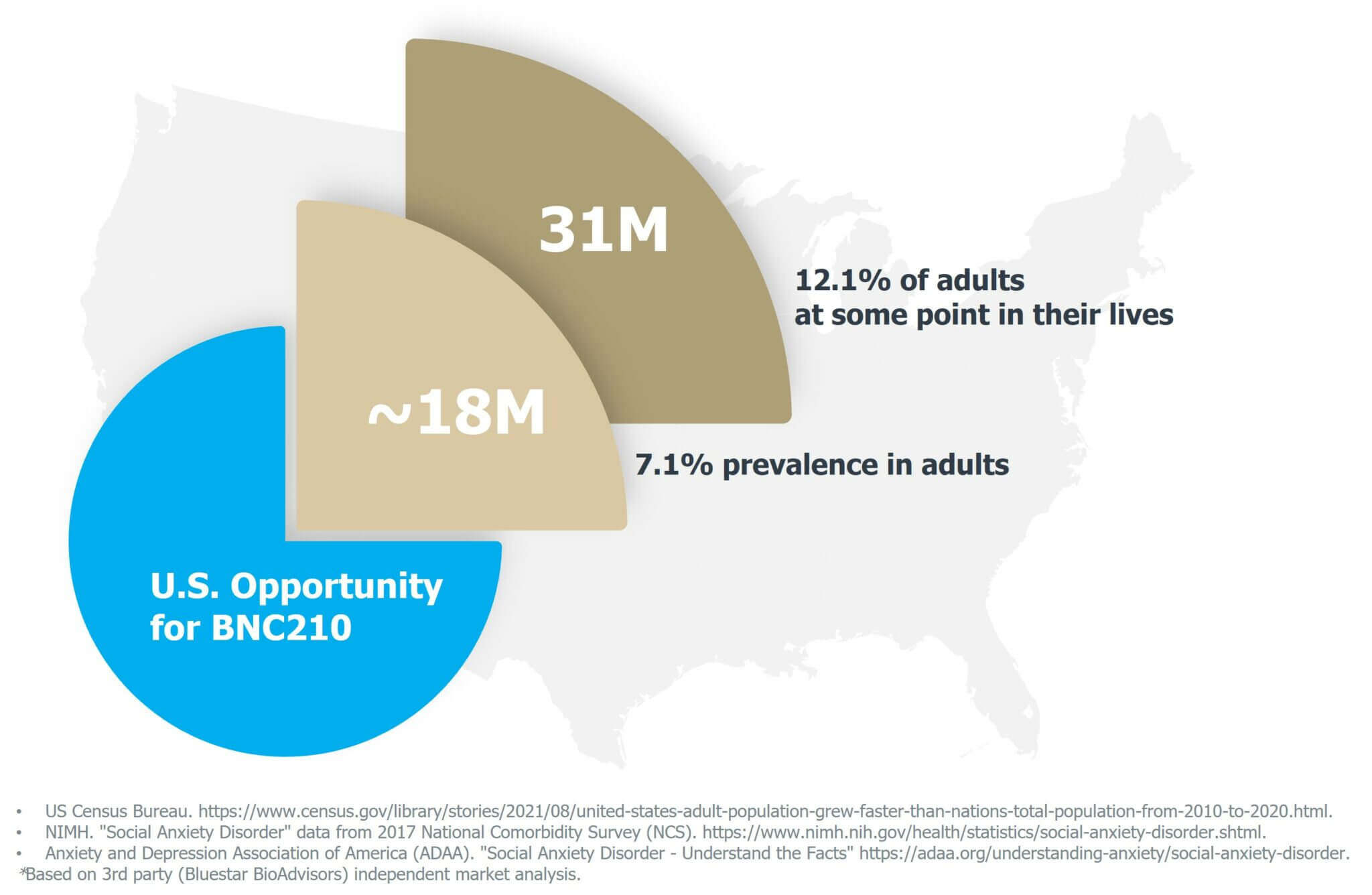
Social Anxiety Disorder is a serious anxiety disorder characterized by the persistent, intense fear of social or performance-related situations in which the person is exposed to unfamiliar people or to possible scrutiny by others. SAD can also be evident in limited settings such as a fear of speaking in formal or informal situations or induced by social interactions across any variety of situations.
For more information on Social Anxiety Disorder, please click on the links below:
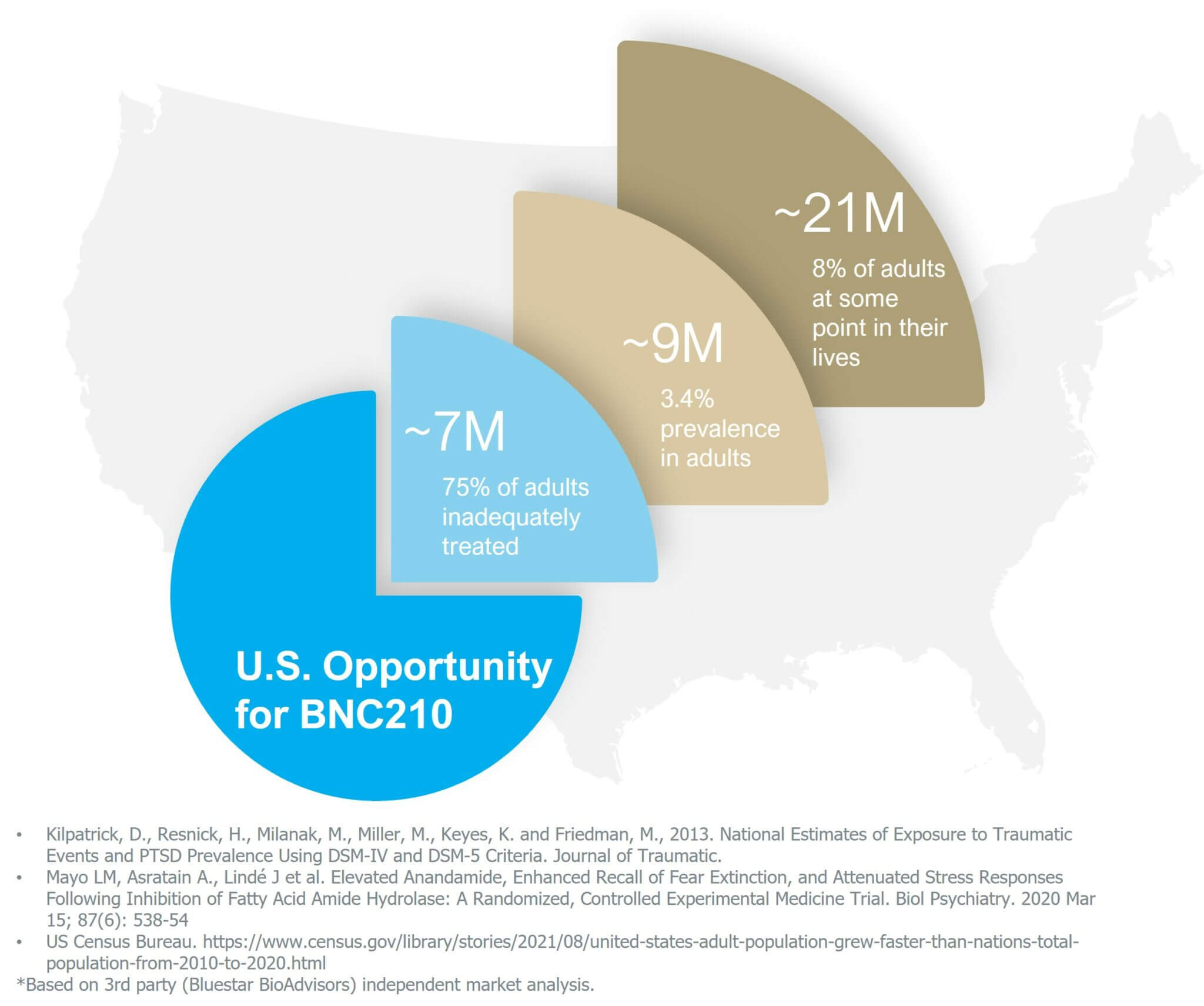
Post-Traumatic Stress Disorder (PTSD)
Post-Traumatic Stress Disorder is a serious, chronic condition triggered by a broad range of severe trauma types such as traumatic experiences in combat experienced by military personnel and other experiences such as childhood trauma and rape. Trauma exposure can trigger a distinctive pattern of persistent, disabling behavioral and physiological symptoms, which include intrusive memories and nightmares of the trauma, severe anxiety, irritability, hypervigilance, depression, difficulty sleeping, poor concentration, and emotional withdrawal.
For more information on post-traumatic stress disorder, please click on the links below:
PAST CLINICAL TRIAL INFORMATION
In clinical studies, BNC210 has exhibited anti-panic properties by reducing the number and intensity of panic symptoms in a human model of Panic Disorder. In patients with Generalized Anxiety Disorder (GAD), BNC210 has demonstrated anti-anxiety activity in a behavioural model of threat avoidance and on anxiety scale scores (STAI). Using functional magnetic resonance imaging (fMRI), BNC210 was shown to reduce hyperactivity in the amygdala of the brain, a region where increased activity is associated with anxiety.
In an initial trial in patients with PTSD, BNC210 did not show a significant effect compared to placebo on key efficacy measures such as the CAPS-5 PTSD scale, but the pharmacometric analyses showed that the amount of BNC210 patients were exposed to in this study was lower than expected and may be part of the reason for this result. Sophisticated statistical modelling suggests that BNC210 has the potential for significant benefit in patients with PTSD, provided that adequate exposures are achieved. A new tablet formulation of BNC210 is in development that in initial trials has demonstrated the ability to achieve the required exposures predicted by the modelling to be potentially effective. Further development is in progress and a second clinical trial in PTSD patients using the tablet formulation of BNC210 is planned for 2021. The development of BNC210 for patients with PTSD using this approach has been given ‘fast-track’ designation by the US Food and Drug Administration (FDA).
Related Media
PARTNERING OPPORTUNITIES
Bionomics has a lengthy history of successful alliances with industry and academia. We are open to expressions of interest with respect to partnership at all stages of development.