Focused CNS Pipeline
Bionomics has a CNS focused pipeline led by its programs in Social Anxiety Disorder (“SAD”) and Post-Traumatic Stress Disorder (“PTSD”), both of which received the U.S Food and Drug Administration (FDA) Fast Track Designation.
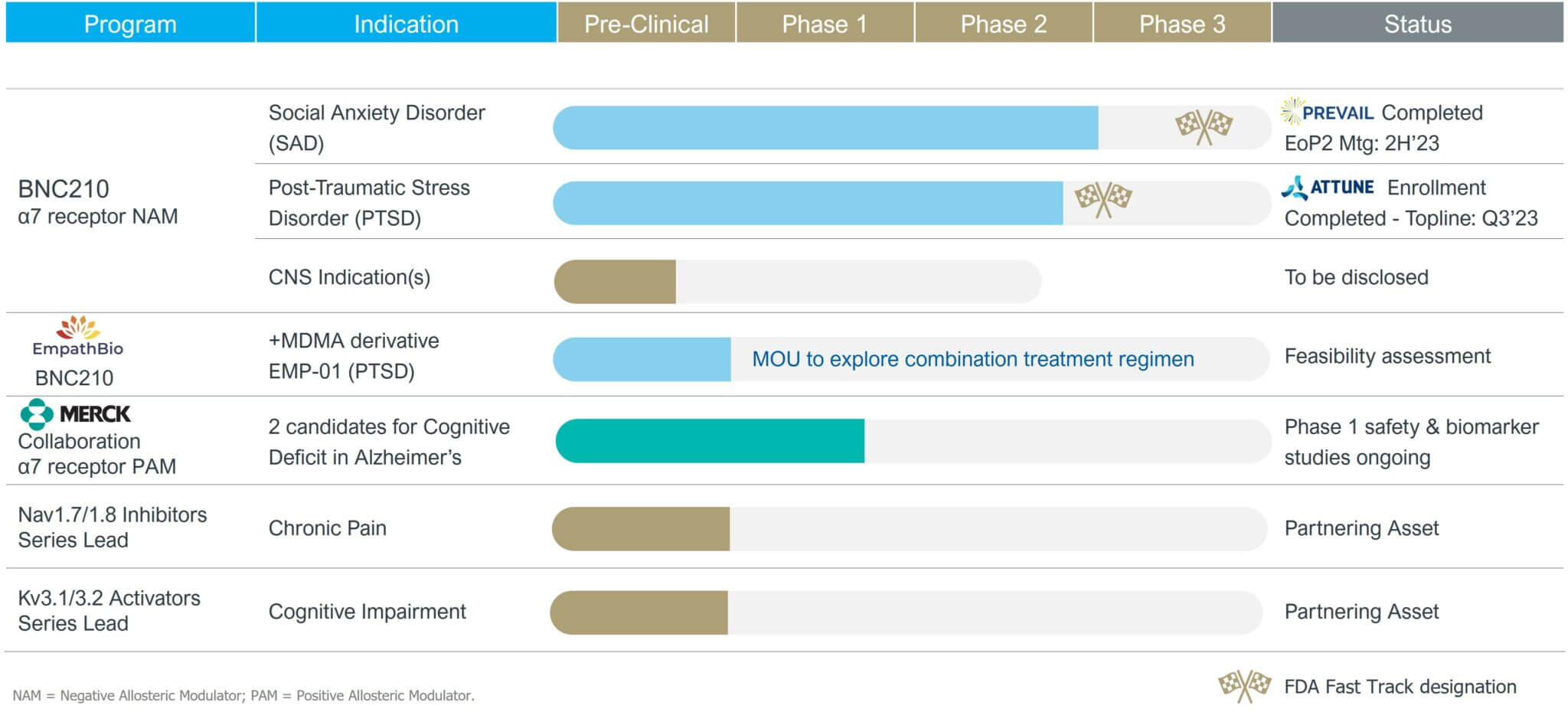
Bionomics has a CNS focused pipeline led by its programs in Social Anxiety Disorder (“SAD”) and Post-Traumatic Stress Disorder (“PTSD”), both of which received the U.S Food and Drug Administration (FDA) Fast Track Designation.

Bionomics conducts clinical trials in various indications. It is the core of what we do.
Learn More